Scientific objectives
-
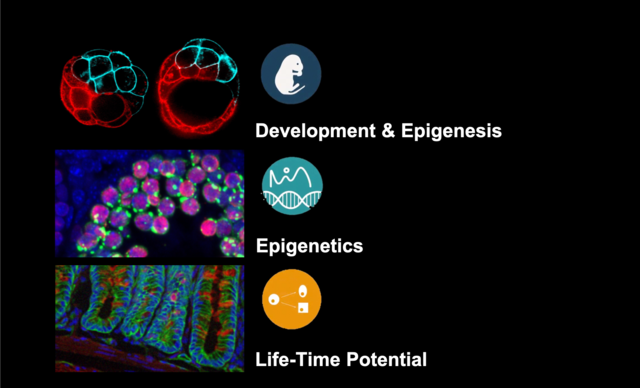
Development & Epigenesis. Analysing how complexity emerges during development
We address several challenging questions in developmental biology: how specific expression patterns are triggered during embryogenesis, how transcriptional changes lead to complex individual and collective cell behaviours and how such changes are integrated with and influenced by epigenetic and mechanical (i.e. physical forces) processes. Tackling these issues requires thorough and quantitative descriptions of transcriptional and epigenomic changes at the single cell level, as well as mechanistic analyses of gene function in space and time during development. Overall, our coordinated efforts address how complexity is progressively established during development in multiple organisms and how information can be integrated from single cells to groups of cells during the development of complex tissues.
-
Epigenetics. Capturing how changes in gene activity and function evolved and can become heritable without changes in DNA sequence
Epigenetic modifications stabilize gene expression patterns and protect genome integrity, with major impact on cell fate. The notion of heritability is also linked to epigenetics: it implies understanding how chromatin organization is maintained during DNA-based processes (replication, repair) and conversely, how it influences these processes. Epigenetic information is also intrinsically flexible and reversible enabling potential reprogramming. Notably, little is known about how environment and metabolism can modulate the epigenome and what happens when the system derails, as observed in diseases. Our coordinated efforts exploiting cutting-edge technologies, including single-cell analyses and single molecule live-microscopy approaches, to study epigenetic mechanisms in a set of model organisms, during the cell cycle and across generations, will provide a sound basis for understanding the mechanisms that influence the stability and plasticity of epigenetic states.
-
Life time potential. Addressing how certain cells maintain the capacity to generate a variety of cells to regenerate tissue
We want to understand how stem cells are maintained throughout life, how they generate and regenerate tissues and how they drive tumor initiation and aging. It is a natural extension of the first research axis, whereby adult tissue growth and stem cell lineages represent the final stages of epigenesis. Taking advantage of in vivo model systems including Drosophila, zebrafish and mouse, as well as in vitro and ex vivo systems including embryonic stem cells and organoid cultures, we are addressing several key questions concerning stem cell proliferation and differentiation, specifically the impact of metabolic programming and reprogramming and chromatin dynamics in these processes, the impact of the environment on tissue homeostasis and the reciprocal impact of stem cell dysfunction in disease and aging. Addressing these questions is paramount and a necessary step towards precision stem cell therapies.

